APT-008
Phase 1 dose escalation and expansion cohort study of EOS-984 as monotherapy and in combination with other anticancer treatments in participants with advanced solid tumors
Overview of Study Design
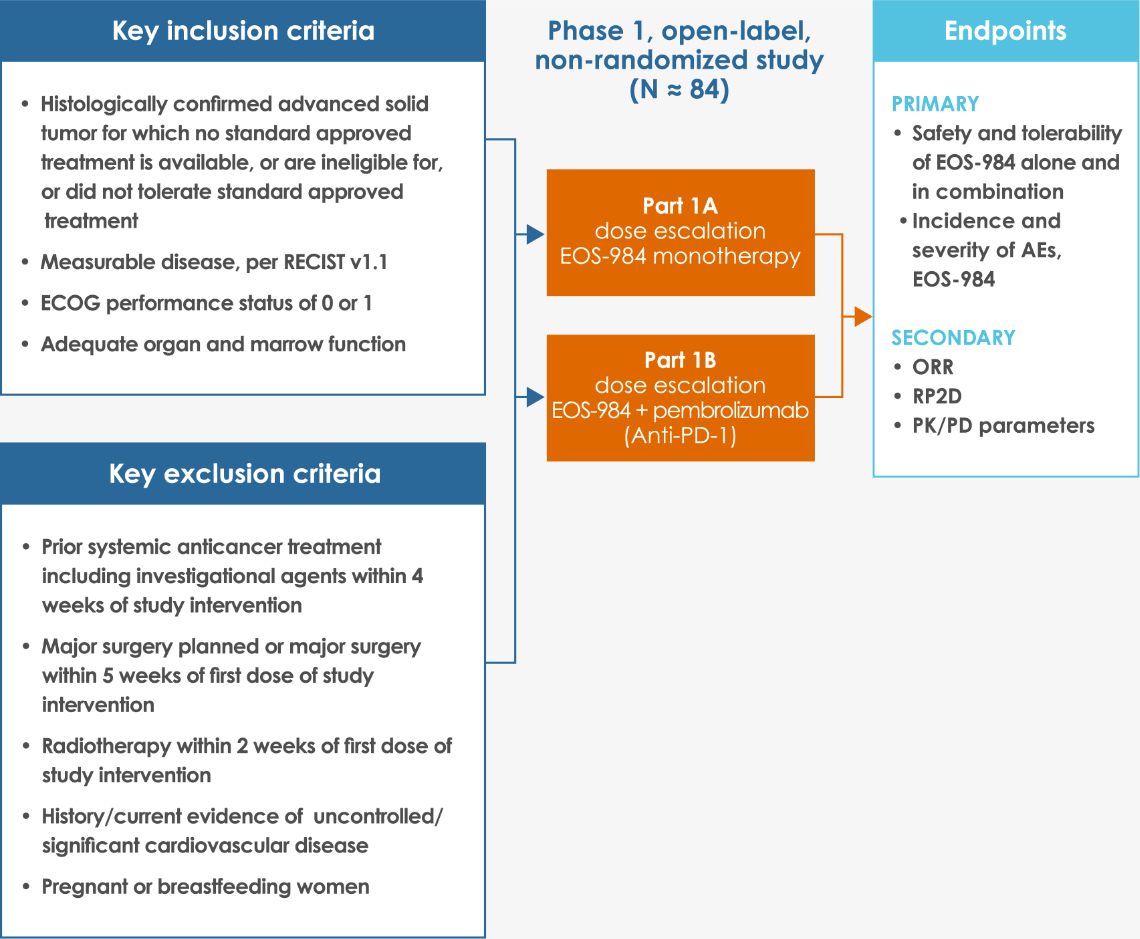
AEs, adverse events; DoR, duration of response; ECOG, Eastern Cooperative Oncology Group; OS, overall survival; ORR, overall response rate; PFS, progression-free survival; PK, pharmacokinetic; PD, pharmacodynamic; PD-1, programmed cell death protein 1; RP2D, recommended phase 2 dose; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1.
